Light calcium carbonate, also known as precipitated calcium carbonate, abbreviated as light calcium, with a molecular formula of CaCO3 and a molecular weight of 100.09. Light calcium is produced by calcining limestone and other raw materials to form lime (mainly composed of calcium oxide) and carbon dioxide, then adding water to digest lime to form lime milk (mainly composed of calcium hydroxide), and then introducing carbon dioxide to carbonize lime milk to form calcium carbonate precipitation. Finally, it is obtained by dehydration, drying, and crushing. Alternatively, sodium carbonate and calcium chloride can be first subjected to a double decomposition reaction to generate calcium carbonate precipitate, which can then be obtained by dehydration, drying, and crushing. Due to the larger sedimentation volume of light calcium carbonate (2.4-2.8 mL/g) compared to heavy calcium carbonate (1.1-1.4 mL/g), it is called light calcium carbonate.
The Properties of lightweight Calcium Carbonate
White powder, odorless, odorless, with a specific gravity of approximately 2.71. Decompose at 825-896.6 º C with a melting point of 1339 º C. There are two forms: amorphous and crystalline, which can be further divided into rhombic and hexagonal crystal systems, forming columnar or rhombic shapes. Difficult to dissolve in water and alcohol. Dissolve in acid and release carbon dioxide, exhibiting an exothermic reaction. Also soluble in ammonium chloride solution. Stable in the air with slight moisture absorption ability.
The Use Of Lightweight Calcium Carbonate
Can be used as a filler in industries such as rubber, plastics, paper, coatings, and inks. Widely used in organic synthesis, metallurgy, glass and asbestos production. It can also be used as a inoculant for industrial wastewater, an acid suppressant for gastric and duodenal ulcer disease, an antidote for acidosis, an SO2 eliminating agent in SO2 containing waste gas, a feed additive for dairy cows, and an anti sticking agent for oil felt. It can also be used as a raw material for tooth powder, toothpaste, and other cosmetics.
The Manufacturing Process Of Lightweight Calcium Carbonate
Carbonization method: Limestone and white coal are mixed in a certain proportion, and then subjected to high-temperature calcination, water digestion, carbon dioxide carbonization, followed by centrifugal dehydration, drying, cooling, crushing, and sieving to obtain the finished product.
CaCO3==high temperature==CaO+CO2 ↑
CaO+H2O==Ca (OH) 2
Ca (OH) 2+CO2==CaCO3 ↓+H2O
Characteristics Of Lightweight Calcium Carbonate Powder
1. The particle shape is regular and can be considered as a monodisperse powder, but it can be in various shapes, such as spindle, cubic, needle, chain, spherical, sheet, and quadrangular column. These different shapes of calcium carbonate can be prepared by controlling reaction conditions.
2. The particle size distribution is relatively narrow.
3. Small particle size, with an average particle size of 1-3 μ M. To determine the average particle size of lightweight calcium carbonate, the short axis particle size in the triaxial particle size can be used as the apparent particle size, and the median particle size can be taken as the average particle size. In the future, unless otherwise specified, the average particle size refers to the average short axis particle size.
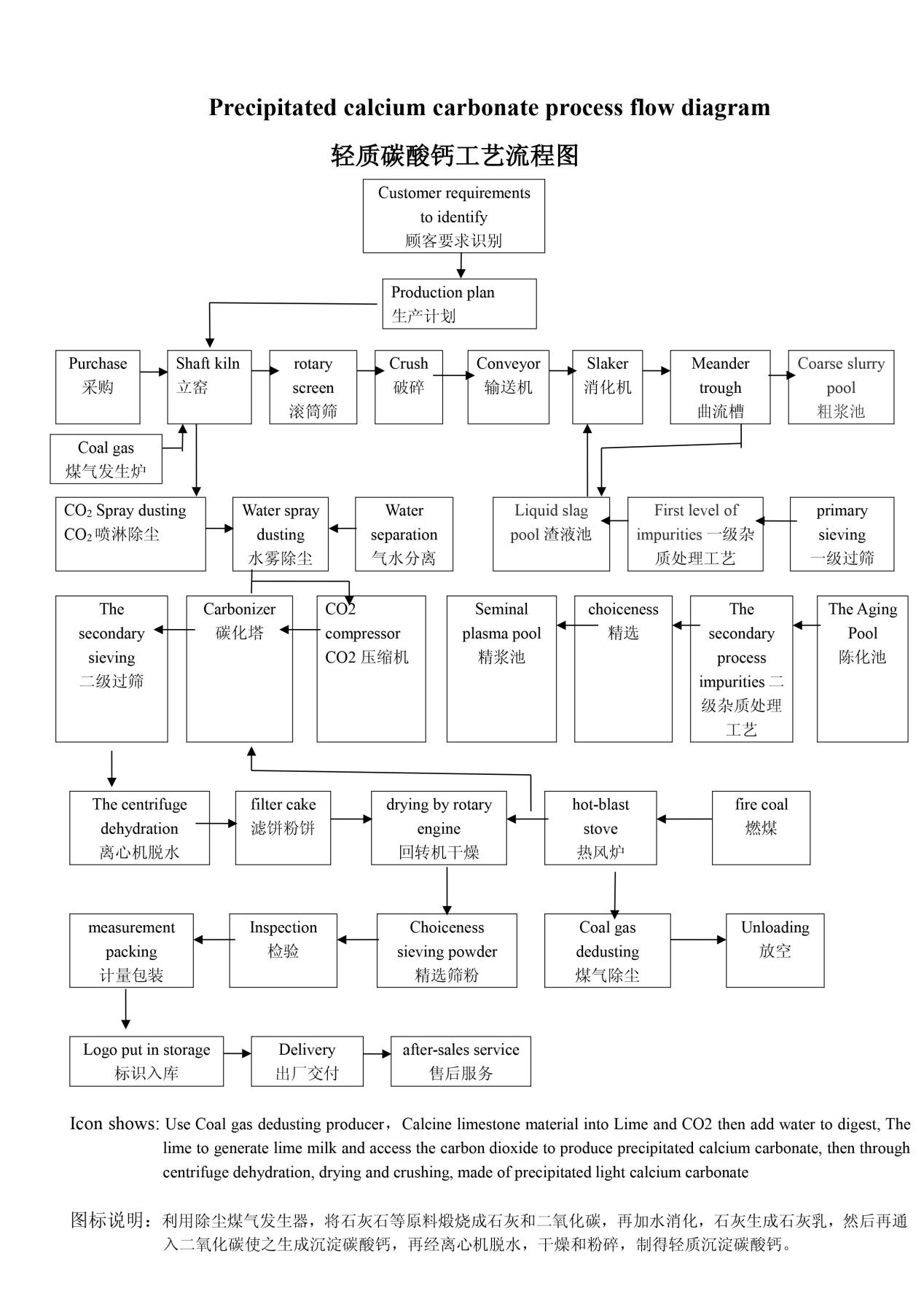
The Process Flow Of Lightweight Calcium Carbonate
1. Limestone calcination: In a lime kiln, limestone and anthracite are added in a 6:1 ratio, and relevant technical calcination auxiliary equipment is used. After the calcination reaction is completed, natural cooling is carried out, and the ash is discharged to obtain quicklime (CaO).
2. Quicklime digestion: Add quicklime and water to the digestion machine (ash machine), dissolve and stir with water, and then the slurry flows into the coarse slurry tank.
3. Carbonization of quicklime water: The lime water in the coarse slurry tank (cement underground tank) is separated (divided into 2 times, 3 times for better effect) before entering the fine slurry tank. Then, the raw slurry from the fine slurry tank is pumped into the carbonization tower (cylindrical carbonization tower) to facilitate the reaction of CO2 from the lime kiln at a temperature of 40-50 ℃. The fine slurry becomes turbid and can react for about 2-3 hours before becoming a cooked slurry. Finally, the cooked slurry is placed in the sedimentation tank.
4. Drying: Use a centrifuge to spin dry equipment for drying, and then proceed to the drying process.
5. Drying: Put the dehydrated powder with 30% moisture into a drying oven and dry it at a temperature of around 200 ° C.
6. Crushing: Using equipment such as a wind separator or airflow screen for screening, the finished product is obtained after crushing.
7. Packaging: Use a packaging machine to pack the finished product into a bag.
8. Storage: Transfer to the warehouse using a forklift.